As the battle against COVID-19 enters month six and cases continue to surge across the country, the search for a vaccine is more urgent than ever. On Monday, June 20, the world received some positive news: A potential coronavirus vaccine developed by Oxford University and pharmaceutical company AstraZeneca has produced a promising immune response in a large, early-stage human trial. Researchers reported that the vaccine produced antibodies and T-cells to combat the infection that lasted at least two months.
Along with this great development, Fenway Health is excited to announce that we will be participating in the next phase of this vaccine trial as a research site. We will be recruiting community members to join this life-saving study and help make the next phase of research successful. We will be releasing more information on this in the coming weeks.
In the meantime, we would like to explain more about vaccine trials and alleviate any concerns potential participants might have about joining this study. We asked Dr. Kenneth Mayer, Co‑Chair and Medical Research Director at The Fenway Institute, to answer some commonly asked questions.
How does vaccine research work?
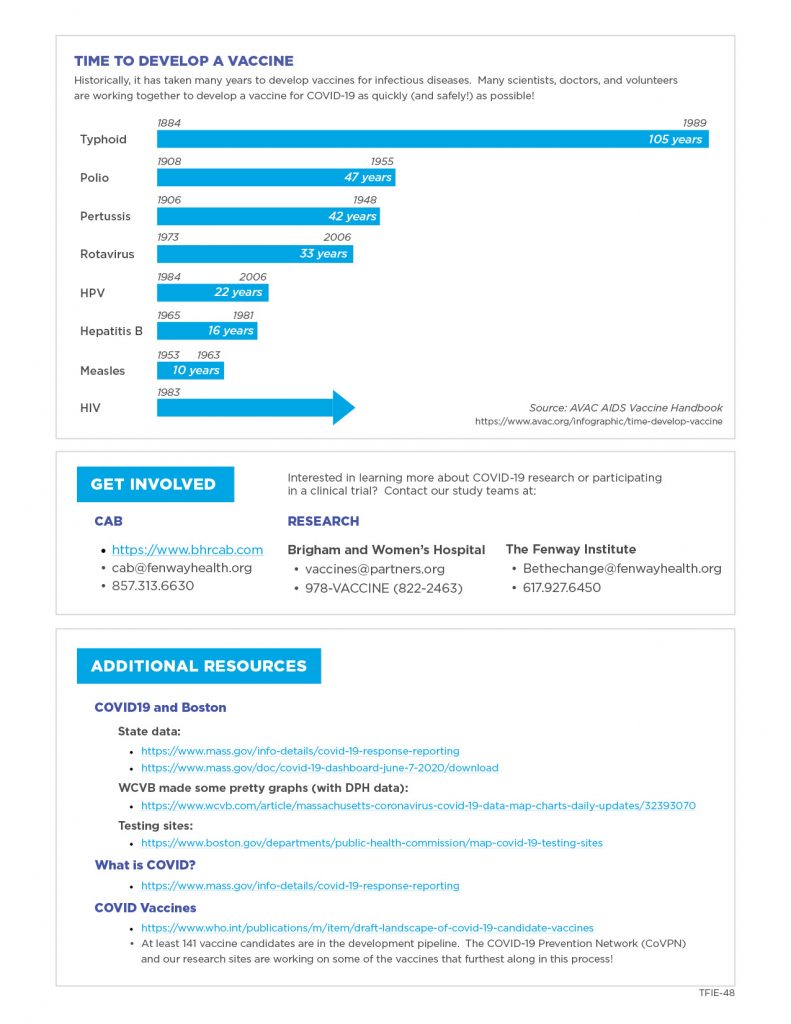
Vaccines are biological substances that trick the immune system into thinking it is encountering an active infection, in order to mount a strong immune response that will protect against the actual infection. Vaccines are first tested in animals for safety and hints that they will work. The first studies in humans are then done in small numbers of low risk people to assess safety. It is then tested in larger numbers of people to see if it consistently stimulates the immune system in ways that would be considered protective. Once the researchers and the federal government are convinced the vaccine is safe and that it stimulates the right kinds of immune responses, it is tested in large numbers of at-risk people to assess whether it can protect against disease.
What are the risks involved in a vaccine trial?
Vaccines are generally safe and well-tolerated. The most common side effects may be soreness in where the vaccine is administered, followed by fever occasionally. Some people may experience flu-like illnesses. These side effects generally last a few days or less. Rare side effects can occur, which can affect any organ system, which is why vaccine trial participants are very carefully monitored. Placebos are used, as some side effects may not be due to the vaccine; if some people experience a side effect and have received the placebo, this tells the researchers that the side effect was not due to the vaccine.

Will study participants be exposed to COVID-19?
The trial will not intentionally expose anyone to COVID-19. The trial will enroll people at risk for COVID-19, such as individuals living in places affected by the pandemic. Some will get the vaccine, and others will receive a matching placebo. The trial will compare the rates of new COVID-19 infection and outcomes in those who got the active vaccine and those who got the placebo. The vaccine trial are being done as quickly as can be done from an ethical and logistical standpoint.
How long will the research trial take to get results?
It is hoped that we will know if some of these new vaccines work by the end of this year, or early 2021.
How will we know if the research trial was successful?
The trials will be monitored by an independent board of scientists, ethicists, biostatisticians, and community members who are not directly part of the trials. They will review the rate of infections in the placebo group vs. the vaccine group in each trial. Once they see a significant trend for protection or lack of protection, they will be able to stop the trial and share the results.
To learn more about this study and see if you are eligible to participate, please email us at [email protected], call us at 617.927.6450, or visit www.coronaviruspreventionnetwork.org.